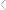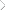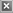Novel Bio-based Poly(vinyl ether)s for Coating Applications
September 2015 www.coatingsworld.com Coatings World | 49
shortly after solvent evaporation from the ;lm. Chemical resistance and ;lm hardness were developed over time due to
crosslinking by autoxidation.
The mechanism of the oxidative process, commonly referred
to as autoxidation, is a free-radical process that possesses initiation, propagation, and termination steps. 3-7 As shown in Figure
1, initiation occurs by abstraction of a bis-allylic hydrogen by
singlet oxygen to produce the carbon-centered radical (I). This
radical is delocalized over the pentadiene structure and reacts
with oxygen to produce the peroxy radical and conjugation in
the fatty acid ester chain (II). The peroxy radical can participate in a number of reactions including hydrogen abstraction
to produce the hydroperoxide (III). The hydroperoxide is thermally unstable and can undergo hemolytic cleavage to produce
an ether radical and a hydroxyl radical (IV). Crosslinks are
formed primarily by radical coupling reactions that result in a
variety of crosslinks including ether bonds, peroxide bonds, and
carbon-carbon bonds.
The general classes of resins/polymers currently used in the
coatings industry include epoxies, polyurethanes, alkyds, acrylics, polyesters, and amino resins. Of these, acrylics represent the
highest volume of resins used in the coatings industry. The utility of acrylic resins can be largely attributed to the tremendous
diversity in thermal and physiochemical properties that can be
achieved through copolymerization. Most coating ;lms derived
from acrylic resins are thermoplastic and thus possess limited
chemical and stain resistance. It has long been recognized that
the incorporation of fatty acid ester chains into the pendent
groups of acrylic resins would be a useful method for introducing crosslinks into coating ;lms to provide enhanced properties.
However, the incorporation of the linoleic and linolenic fatty
acid esters needed for effective crosslinking into an acrylate or
methacrylate monomer would be expected to be problematic
due to the presence of the readily extractable bis-allylic hydrogen atoms. These bis-allylic hydrogen atoms would be expected
to lead to extensive chain transfer and perhaps gelation during
the polymerization. Further, radical addition to double bonds
present in the fatty acid ester chains could also lead to gelation
during polymerization.
In the last few decades, tremendous progress has been made in
the carbocationic polymerization of vinyl monomers. 8 Although
carbocations are generally very reactive species, polymerization
processes have been developed that enable very controlled po-
lymerization. In fact, living carbocationic polymerization sys-
tems have been developed for a number of monomers including
vinyl ethers, isobutylene, and styrene. The controlled reactivity
of the propagation step with these living polymerization systems
is generally believed to be the result of a propagation-step that
involves an equilibrium between dormant and active species.
In addition, the active carbocation is stabilized through inter-
actions with the counter anion and/or interactions with Lewis
base additives present in the polymerization system. A number
of polymerization variables can be used to tailor the nature of
a carbocationic polymerization including temperature, initiator
composition, Lewis acid coinitiator composition and concentra-
tion, addition of a Lewis base, Lewis base composition and con-
centration, and solvent composition. This document provides
an overview of a polymer technology that enables the produc-
tion of novel poly(vinyl ether)s based on renewable materials
and their application as coatings binders.
Polymers Based on Plant Oils
Using simple base-catalyzed transesteri;cation of a vinyl ether
alcohol with either a plant oil triglyceride or fatty alkyl ester, a
novel vinyl ether monomer was produced. 9 Figure 2 shows the
synthetic process using 2-(vinyloxy)ethanol as the vinyl ether
alcohol and methyl soyate as the fatty alkyl ester. As illustrated
in Figure 2, this monomer, 2-(vinyloxy)ethyl soyate (2-VOES), is
a mixture based on the fatty acid ester composition of methyl
soyate. As illustrated in Figure 3, the polymerization system developed for these plant oil-based vinyl ether monomers involves
the use of the addition product of isobutyl vinyl ether and acetic
acid as the initiator, ethylaluminum sesquichloride as the coinitiator, and toluene as the solvent. Using this system, a living
polymerization was achieved. 10
For most carbocationic polymerizations of a vinyl ether produced using a Lewis Acid coinitiator, such as ethylaluminum
sesquichloride, an appropriate concentration of Lewis base is
needed to obtain a living polymerization. The mechanism of
‘Lewis-base assisted living cationic polymerization’ is believed
to involve an equilibrium between dormant and active chain
ends with the concentration of active chain ends being much
lower than that of the dormant chain ends. The Lewis base
is believed to reduce both the concentration and the reactivity
of active chain ends. As described by Kanazawa et al., 11, 12 the
Lewis base: (1) complexes with the Lewis acid coinitiator resulting in the formation of monomeric Lewis acid species and an
adjustment of acidity; (2) stabilizes active chains through direct
interaction; and ( 3) stabilizes the counteranion generated upon
initiation. For the polymerization of 2-VOES, it is believed
that the ester group present in the monomer serves the role of a
Lewis base additive typically utilized in a ‘Lewis base-assisted’
living carbocationic polymerization. Obtaining a living polymerization enabled control of polymer molecule weight, narrow
molecular weight distribution polymers, and the production of
block copolymers. 13
Figure 1. Schematic illustrating the autoxidation process.