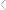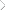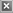Novel Bio-based Poly(vinyl ether)s for Coating Applications
September 2015 www.coatingsworld.com Coatings World | 53
As shown in Figure 9, the presence of the vinyl groups in
poly(EEVE) enables the production of polyepoxide resins by
simple oxidation using, for example, m-chloroperoxybenzoic
acid. An epoxidized version of poly(EEVE) was produced and
crosslinked ;lms generated using diethylenetriamine (DETA) as
the crosslinking agent and a 1/1 epoxy/NH ratio. For comparison purposes, crosslinked ;lms of the diglycidyl ether of bisphe-nol-A (DGEBPA) were also produced using DETA. Figure 10
displays the viscoelastic properties of the network derived from
epoxidized poly(EEVE) and DETA. From the tangent delta data,
a Tg of 130 °C was determined. It was very interesting to observe this high of a Tg considering the relatively high molecular
mobility of the poly(vinyl ether) polymer backbone. Obviously
the very high crosslink density derived from the high number
of epoxy groups per polymer molecule enables such a high Tg.
Table 3 provides a comparison of the properties of coat-
ings cast and cured on steel substrates. As shown in Table 3,
the hardness, ;exibility, and adhesion of the coating based on
epoxidized poly(EEVE) was similar to that of the analogous
coating based on DGEBPA. The primary difference between
these two coatings involved the chemical and impact resis-
tance. The chemical resistance, as expressed using the MEK
double rub test, was dramatically better for the coatings
based on epoxidized poly(EEVE). After 1,000 MEK double
rubs, no visible damage to the coating was observed. In con-
trast, the coating based on DGEBPA failed after 310 double
rubs. With regard to impact resistance, the coating based
on the epoxidized poly(EEVE) showed a lower impact resis-
tance than the coating based on DGEBPA. The higher MEK
resistance and lower impact resistance associated with the
epoxidized poly(EEVE) is consistent with a higher crosslink
density for this coating.
Conclusion
The results presented in this document demonstrate the utility of a carbocationic polymerization system for producing
novel bio-based polymers that retain the unsaturation derived from the bio-based component. The high reactivity of
the vinyl ether functional group and the appropriate choice
of the polymerization system enabled linear polymers to be
produced with relatively narrow molecular weight distributions. The ability to retain unsaturation from the bio-based
component enabled the production of crosslinked coatings
using autoxidation. The high number of allylic hydrogens
and double bonds per molecule associated with these unsaturated poly(vinyl ether)s results in relatively fast curing by
autoxidation due to the gel-point being reached at relatively
low extents of reaction. Another important aspect of this
polymer technology is the ability to utilize copolymerization
to tailor polymer and coating properties. The unsaturation
present in the polymer produced can also be easily converted
to other functional groups, such as the epoxy group, to enable other crosslinking mechanisms. CW
Acknowledgement
The authors thank the Department of Energy (grant DE-
FG36-08GO088160), United States Department of
Agriculture/National Institute of Food and Agriculture
(grant 2012-38202-19283), United Soybean Board, National
Science Foundation (grants IIA-1330840, IIA-1355466, and
IIP-1401801), and North Dakota Soybean Council for ;nan-cial support.
Figure 9. Synthetic scheme used to produce an epoxidized version of
poly(EEVE).
Figure 10. Viscoelastic properties of the network derived from epoxidized
poly(EEVE) and DETA.
Table 3. Data for coatings based on epoxidized poly(EEVE) and DGEBPA cast
and cured on steel substrates.