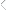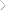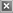If the sorption profile of the reactant into the coating is
not well understood, it is not possible to accurately characterize the latent functionality, activity and reten-tion/optimization characteristics of the functional coating.
In one example, it was determined that reactant sorption
must be considered when selecting the most efficacious
polymer type so that the decontamination activity of OPH
enzymes matches the environmental conditions under
which the coating will be expected to function. A study of
the performance of the OPH enzyme in a number of different polymeric systems demonstrates the importance of
careful coating binder selection. 25 Optimum functionality
of the immobilized biocatalyst in this system can be
achieved when the polymer closely matches the solubility
parameters of the reactant, which ensures a reduction in
diffusional constraints and enables saturation of the
enzyme active site. Engineering functional coatings with
latent, stable, extended film-life catalytic capabilities clearly requires an understanding of the many physical and
chemical relationships that exist between the resin, the
immobilized biocompound and the reagents to be acted
upon under the expected environmental conditions (see
Figures 7 a & 7b).
The impact of polymer composition can also clearly be seen
in the case of embedded lipase enzymes. To screen for compatibility with polymer systems, Reactive Surfaces blended
enzymes with solution phase polymers and then assessed
the biocatalytic properties. 23 In general, lipase performed
well in most of the tested resin systems, with the exception
of the isocyanate system. Based on these results, the acrylic
resin system (Avanse MV- 100) was selected for formation of
biocatalytic free films. Importantly, it was observed that the
catalytic rate of hydrolysis increases with increasing surface
area but is independent of bulk volume (compare DeGreez
lipase in Figure 8a to OPDtox hydrolase in Figure 8b).
While performance is a critical issue, the potential
impact of embedding the desired biocompound into a coating formulation on key coating characteristics is equally
important. The modified paint or coating must continue to
deliver desired physical properties such as gloss, hardness,
adhesion and impact resistance. Evaluation of these
parameters must also be completed in order to present a
comprehensive assessment of coating performance.
In the case of lipase incorporation, Reactive Surfaces
found that addition of the enzyme had no discernable effect
on coating performance at levels of 3% and 14.3%, with all
properties measured equaling the unmodified standard. 23
With regard to chemical resistance testing, at the lower
level of enzyme addition there was minimal effect on water
and aqueous solution exposures with the only notable
effects being observed with the 10% sodium hydroxide
exposure and the red wine stain exposure. At high enzyme
loading, greater film softening and varying degrees of blistering were observed upon exposure to water and aqueous
acid, alkaline and chloride solutions when compared to the
standard. Work is ongoing to improve the enzyme formulation in order to minimize the effects observed at higher
enzyme loading levels.
Once an effective bioactive/polymer composition has been
identified, the final step in the development of reactive
coatings must include evaluation of activity under simulated application conditions. For example, to test the lipase
modified coating, panels were coated with the lipase-con-taining formulation and heavily contaminated with a thick
layer of vegetable oil. During a period of 72 hours, the coating cleared the surface of all oil. 23 DeGreez has also been
shown to remain active in kitchen coatings after 100 and
200 scrub cycles using ASTM D3207.
These phases of biofunctional coating development can
Figures 8a & 8b
(a)
(b)
(a) DeGreez hydrolysis of p-nitrophenol acetate shows
hydrolysis rate is not affected by bulk volume (film thickness);
(b) OPDtox hydrolysis of paraoxon shows hydrolysis rate is
affected by bulk volume